Following public consultation and official endorsement, the final version of the RDA COVID-19 Recommendations and Guidelines for Data Sharing was published today, 30 June 2020.
- Research Data Alliance COVID-19 Recommendations and Guidelines on Data Sharing Final Executive Summary 30 June 2020
- Research Data Alliance COVID-19 Recommendations and Guidelines on Data Sharing Final Release 30 June 2020
- Research Data Alliance COVID-19 Working Group
- Research Data Alliance COVID-19 All Working Group Outputs (5 Releases) – Draft releases and comments available here: DOI: https://doi.org/10.15497/rda00046
Data holds the potential to drive rapid response and informed decision-making during public health emergencies. There is a need for timely and accurate collection, reporting and sharing of data within and between research communities, public health practitioners, clinicians and policymakers. Accurate and rapid availability of data will inform assessment of the severity, spread and impact of a pandemic to implement efficient and effective response strategies.
The availability of efficient information and communication technology has improved the global capacity to implement systems to share data during a pandemic. However, the harmonisation across these sophisticated yet diverse systems combined with the timeliness of accessing data across information systems are currently major roadblocks.
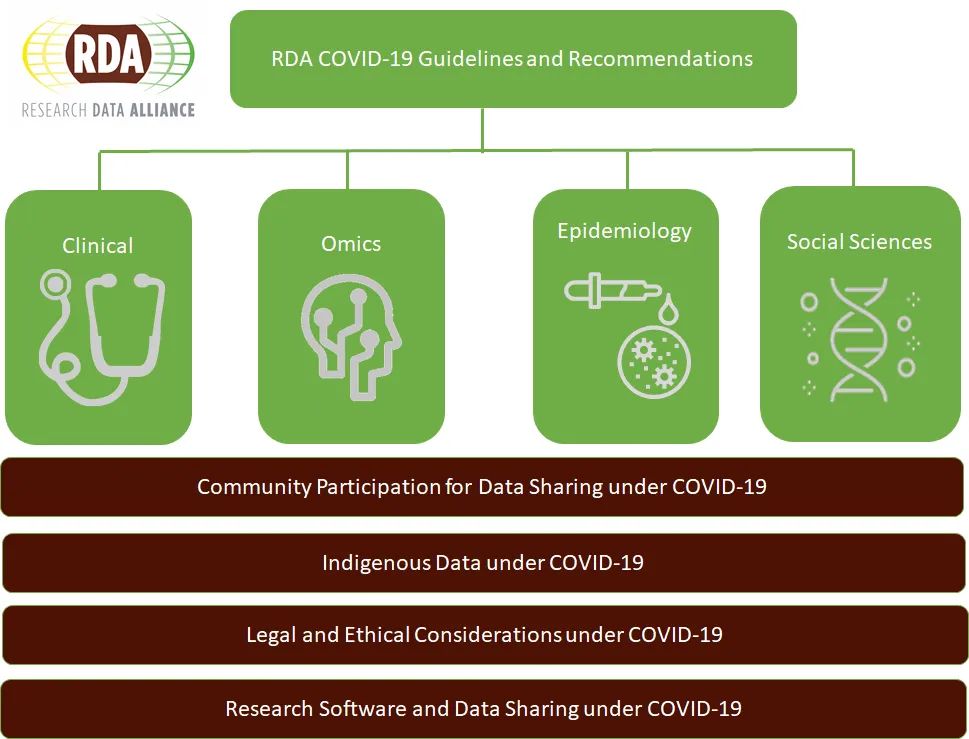
The RDA COVID-19 Working Group (CWG) was created as a response to the challenges posed by data sharing in the midst of a pandemic. The members have brought varied global expertise to develop a body of work that comprises how data from multiple disciplines inform response to a pandemic combined with guidelines and recommendations on data sharing under the present COVID-19 circumstances. This extends to research software sharing, in recognition of the key role played by software in analysing data. The work has been divided into four research areas (Clinical, Omics, Epidemiology, Social Sciences) with four cross-cutting themes (Community Participation, Indigenous Data, Legal and Ethical Considerations, Research Software), as a way to focus the conversations and provide an initial set of guidelines in a tight timeframe. The detailed guidelines are aimed to help stakeholders follow best practices to maximise the efficiency of their work, and to act as a blueprint for future emergencies. The recommendations in the document are aimed at helping policymakers and funders to maximise timely, quality data sharing and appropriate responses in such health emergencies.
This work was executed in an intense period over just over 6 weeks, with five iterations, all of which were opened for public community comment. Draft releases and comments are available here (https://doi.org/10.15497/rda00046).
This activity has been conducted under the RDA guiding principles of Openness, Consensus, Balance, Harmonization, Community-driven, and Non-profit and technology-neutral.
____________________
DRI is honoured to be making direct contributions to this effort. Natalie Harrower is a Co-Chair of the RDA COVID-19 Working group, lead of the Editorial team, and a member of the Epidemiology sub-WG. Timea Biro is a moderator for the Community Participation cross-cutting theme group.
____________________